- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
On July 12, 2023, the European Commission issued the amending regulation (EU) 2023/1442 of the plastic regulation (EU) 10/2011 for food contact. The main amendments include revoking two permitted substances, tightening the requirements for phthalate plasticizers, modifying the use requirements of permitted substances and adding new permitted substances. The new regulationcame into force on August 1, 2023.
Revoke two permitted substances
The new regulation revokes the permission of FCM substance No. 96 (untreated wood flour and wood fibers) and FCM substance No. 121 (salicylic acid, CAS No0000069 - 72 - 7).
Tighten the requirements for phthalate plasticizers
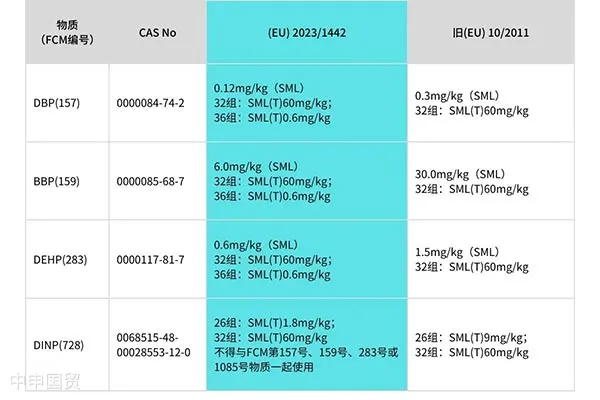
1、加嚴了DBP、BBP、DEHP、DINP的限制性要求,并在第7組中增加了FCM1081號物質。
The SML(T) limit of Group 26 is reduced from 9mg/kg to 1.8mg/kg.
Substances FCM1078 and 1085 are added to Group 32. A special note is made on the use of DIBP of substance FCM1085: DIBP is not listed as a permitted substance in Table 1 of Annex I of Regulation (EU) No10/2011. Since it is used as a polymerization aid, it may co - exist with other phthalates. Therefore, DIBP is included in the restrictions of Group 32.
Three new group numbers from 36 to 38 are added, and the calculation formula and limit of the sum of four phthalates (DBP, BBP, DEHP and DIBP) are specified. The sum is calculated as DEHP, and the calculation formula is: DBP×5 + DIBP×4 + BBP×0.1+DEHP×1, SML(T)=0.6mg/kg.
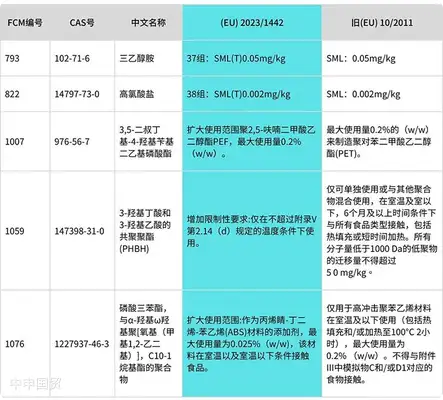
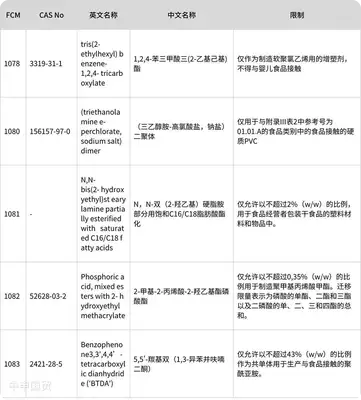
Transitional provisions
The new regulation will come into force on August 1, 2023. Plastic materials and products that comply with the (EU) 10/2011 regulation applicable before the entry into force of the new regulation and are first placed on the market before February 1, 2025, can continue to be sold on the market until the inventory is used up.
For plastic materials and articles manufactured using salicylic acid (FCM121) or using untreated wood flour and fibers, there are special transitional provisions. Under specific conditions, they can continue to be first placed on the market after February 1, 2025.
- Before August 1, 2024, a permission application for the substance has been submitted to the competent authority in accordance with Article 9 of Regulation (EC) 1935/2004;
- Plastic materials and articles manufactured using this substance are limited to the intended use conditions stated in the application;
- The information provided to the authority in accordance with Article 9(1)(b) of Regulation (EC) 1935/2004 includes a statement indicating that the application is made in accordance with this paragraph;
- The authority considers the application to be valid.
Related Recommendations
? 2025. All Rights Reserved. 滬ICP備2023007705號-2  PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912










